When you think about what a molecule is, you may well imagine a picture like this one.
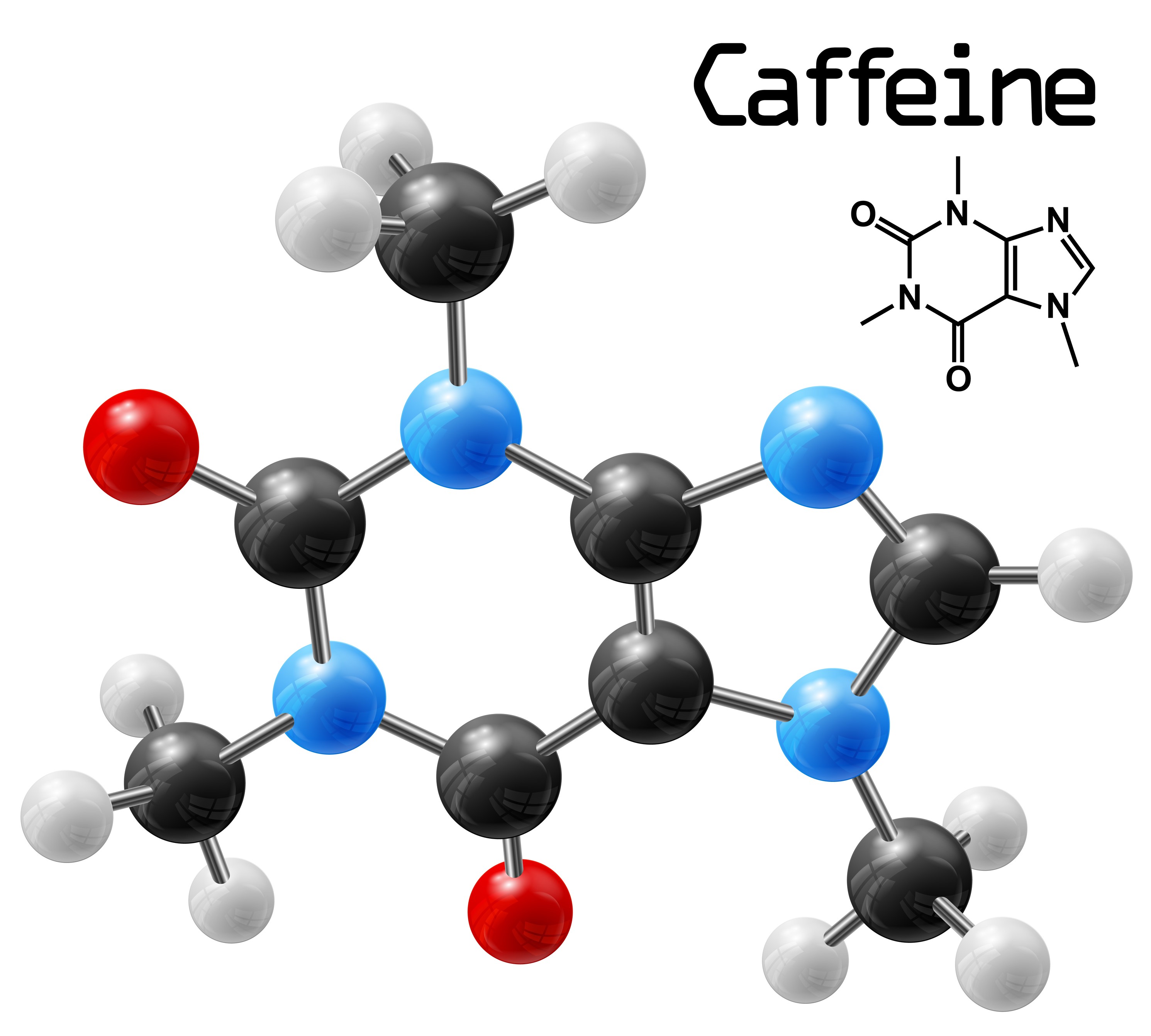
In an image like this, atoms, represented by coloured balls, are joined together by sticks. The types and numbers of atoms, and the way in which the atoms are joined, is different for each different molecule. The one in the picture is caffeine, the molecule that keeps you awake if you drink too much coffee.
But how do we know what this molecule looks like? Atoms and molecules are VERY small – one caffeine molecule is only about 0.000000001 m wide!This is much too small for us to see with our eyes. But to understand how chemists can see a caffeine molecule, we must first understand a bit about how we see normal sized things.

When we look at, say, an apple, the reason that we can see it is that light from the sun (or a light bulb if you are inside) bounces off the apple and some of the scattered light rays hit your eye. These light rays are recombined into an image by the lens in the front of your eye and the image is then interpreted by your brain as being an apple.
If you want to look at something smaller, say an ant, then you might need to use a magnifying glass or a microscope. These have more powerful lenses, and extra lights, to catch the smaller amounts of scattered light that come from the smaller object.
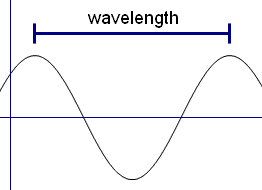 Light rays have a property called wavelength. We say that visible light is when the rays have wavelengths of between 4x10-7 m (0.0000004 m) and 7x10-7 m (0.0000007 m) It turns out that to see an object in the way we have just talked about, the object need to be bigger than the wavelength of the light – that is the object needs to be bigger than 7x10-7 m. But molecules are about 100 times smaller than this. So there is no way we can see molecules using visible light.
Light rays have a property called wavelength. We say that visible light is when the rays have wavelengths of between 4x10-7 m (0.0000004 m) and 7x10-7 m (0.0000007 m) It turns out that to see an object in the way we have just talked about, the object need to be bigger than the wavelength of the light – that is the object needs to be bigger than 7x10-7 m. But molecules are about 100 times smaller than this. So there is no way we can see molecules using visible light.
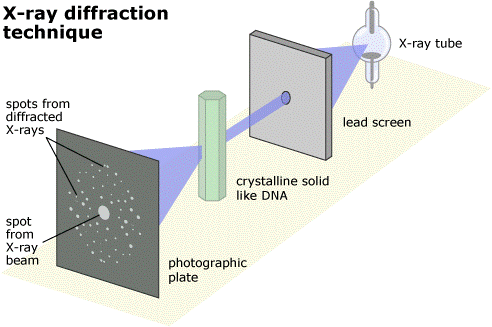
The solution is to use a type of light that has a much smaller wavelength - one that is about the same size as molecules. It turns out that the type of light with this wavelength is X-rays.
But there is another problem. You can shine X-rays onto an object and they will bounce off it (or diffract, to use the correct word) but you can't build lenses to recombine the scattered rays into an image, in the way you can with visible light. And a second problem is that X-rays can be quite dangerous and would be very bad for your eyes if you looked straight into a beam of them. For these reasons, you can't build an X-ray microscope to look at molecules.
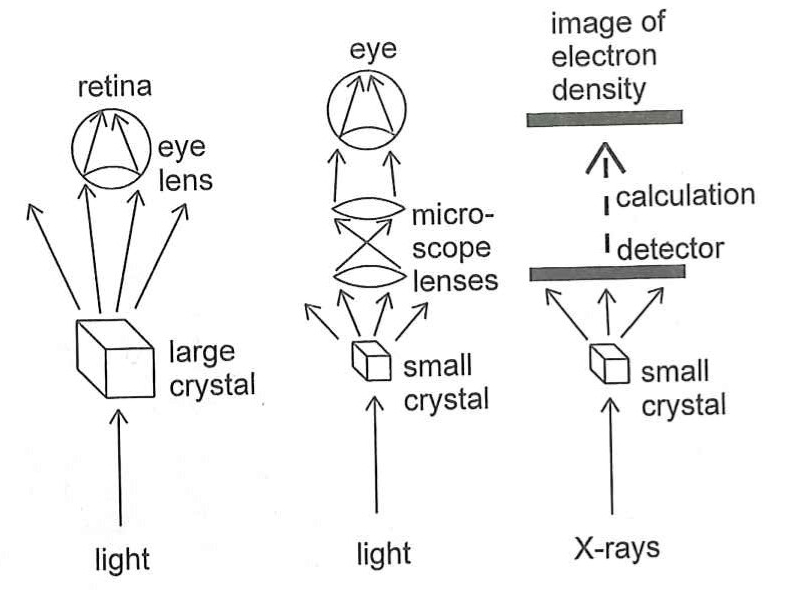 However, instead of using lenses to actually recombine the scattered X-rays, what you can do instead if recombine them in a computer using maths (quite complicated maths!) And this is what X-ray Crystallography is fundamentally about – shining X-rays at a molecule, seeing how they are scattered and then using mathematical equations to recombine them into an image of the molecule.
However, instead of using lenses to actually recombine the scattered X-rays, what you can do instead if recombine them in a computer using maths (quite complicated maths!) And this is what X-ray Crystallography is fundamentally about – shining X-rays at a molecule, seeing how they are scattered and then using mathematical equations to recombine them into an image of the molecule.
But what about the 'crystal' part?
Well the problem is that the amount of X-rays that would be scattered off just one molecule would be so tiny that they couldn't be measured accurately. What you want instead is a huge number of molecules that are all arranged in exactly the same way, so that X-rays scattered off them would be just like the scattering off only one.
And this is where the crystal comes in, because in a crystal this is exactly what you have – all the molecules lined up in a very carefully arranged way.

Sometimes crystals grow in such a way that more than one crystal (with all the molecules lined up in one direction) grow inside another crystal (where all the molecules are lined up in a different direction). This is called a 'Twinned' crystal, and these are no good for our X-ray analysis. Instead, we need what is called a 'Single' crystal - one that only has the molecules pointing one way.
Sometimes, a twinned crystal, like this one, can be cut up to get a single crystal (the ends of these one look like they would be single and could be used for the experiment)
Growing single crystals of the molecule you are interested in can take a lot of time and lots of experimenting with different ways before you get crystals that are ok to use.
So how does it work?
The experiment works like this. First, a single crystal is placed on something called a Goniometer Head. The crystal can't be any bigger than about 0.5 mm long, so picking the crystal and attaching it to the goniometer head is done using a microscope.
Then the goniometer head with the attached crystal is placed inside the X-ray Diffractometer instrument.
When the machine starts, it shoots a beam of X-rays at the crystal. Where the X-rays are scattered to is then measured by a special camera. The resulting photo has a number of bright spots on it, each corresponding to where a scattered X-ray hit the camera. Each photo takes about 15 seconds.
The crystal is then rotated by half a degree and another photo is taken. As the crystal is rotated all the way around, 720 photos are taken in total. The crystal is then moved to a new orientation and another set of photos are taken. In all, about 2000 X-ray photos are needed.
Then the maths kicks in. Powerful computers measure the positions of the scattered X-rays, and then work out where the atoms must have been in the crystal to make the X-rays scatter in the way that they did. By measuring how bright the spots are, it can also work out what sorts of atoms were doing the scattering (oxygen, carbon, nitrogen, etc).
Here are pictures of what an X-ray diffractometer looked like in 1934, and then one of the machines that is at the University of Otago Chemistry Department.

