Introduction
This antimony section of the Otago Geology website is taken directly from a poster presented at the International Sb Workshop inHeidelberg, Germany, May 2005. The authors are P.M. Ashley 1, D. Craw2 & M. Tighe1, the title is given above.
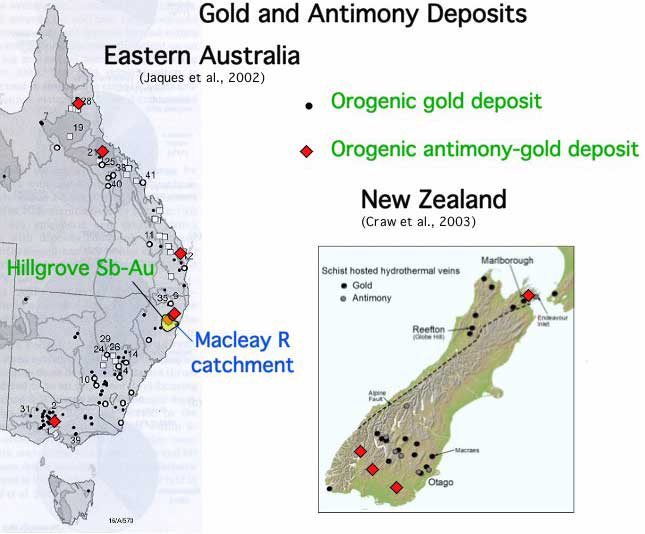
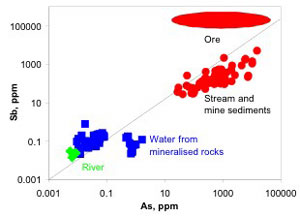
Historic mining practices at several Sb-rich and Sb-bearing orogenic gold deposits in eastern Australia and the South Island of New Zealand have led to significant environmental contamination of streams. The mineral deposits contain stibnite and Sb-bearing arsenopyrite as primary minerals, with small amounts of Sb oxides, Fe oxyhydroxides and scorodite in the oxidised zones.
Although natural weathering and erosion processes have led to Sb anomalies in stream water and sediments, unconstrained disposal of mine wastes has led to extensive contamination of water and sediments, in some cases for distances of tens to hundreds of kilometres downstream. Antimony dissolves readily, along with As, in near-neutral stream waters, most likely as SbO3-. Detrital stibnite is gradually dissolved and subject to attrition, with transfer of Sb into solution and precipitated into hydrated iron oxides (HFO), clays, organic material and neoformed sulphides.
In the fluvial environment, Sb can be mobilised and cycled through aquatic ecosystems and riparian vegetation. In the most contaminated streams, water and sediment quality are severely compromised and greatly exceed national guideline values.
Study Sites
Studies have been performed on the environmental geochemical effects of mining and processing of Sb-bearing ores on stream systems at Hillgrove and Mungay Creek (NSW, Australia) and Reefton, Endeavour Inlet and in Central and Eastern Otago (South Island, New Zealand). At most of these sites, the host rocks to mineralisation are low grade metamorphosed sediments, although granitoids also occur at Hillgrove.
Mineralisation is typified by quartz veins and breccia containing low total sulphide concentration, with stibnite, arsenopyrite and pyrite dominating, accompanied by carbonate minerals and gold. Oxidation of ores does not yield acid mine drainage, as incipient acid production is buffered by carbonates; drainage water typically has pH values of 6.0-8.5.
At each location, the climate is temperate, although rainfall varies from 2000 mm/year to 600 mm/year and evaporation varies approximately inversely. Surface stream water flow is commonly perennial.
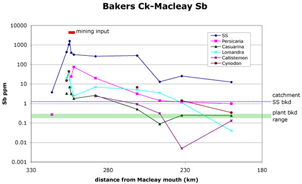
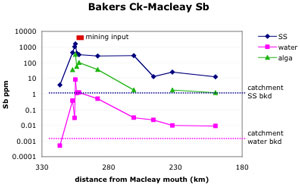
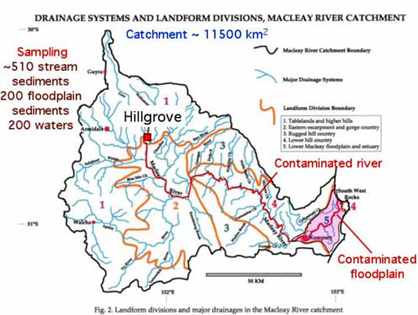
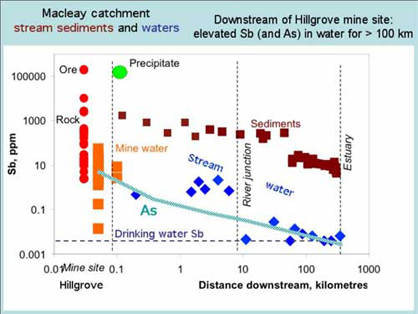
The Hillgrove site has been the major focus as it contains the largest Sb-mining region in Australia. Historic mine waste disposal practices have caused extensive Sb and As contamination of water and sediment in the Bakers Creek-Macleay River system, for 300 km downstream to the Pacific Ocean.
Hillgrove and Macleay River catchment, NSW
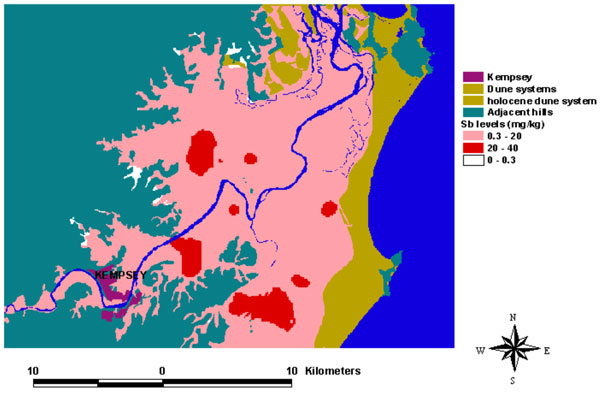
Contamination derived from mine waste at Hillgrove has affected stream sediment quality for over 300 km in the Macleay River system to the Pacific Ocean. Much of the stream system is of high-energy type (gravel bed) until the floodplain. Periodic flooding has dispersed contaminated sediment across the floodplain.
National sediment guideline values for Sb are exceeded (ISQG-low = 2 ppm Sb, ISQG-high = 25 ppm Sb). The catchment Sb background value in stream sediments is 1.1 ppm. Water quality is greatly affected for 20 km (dissolved Sb averaging 1 mg/L), with values locally exceeding drinking water quidelines (3 µg/L) to the ocean. Arsenic values in stream sediments and in water are similar to those of Sb.
Drainage systems and landform divisions
Antimony concentrations

1900's mining

Mine waste

Remnants of historic mine dump adjacent to Bakers Creek.

HFO with up to 11% Sb and 10%
Macleay floodplain
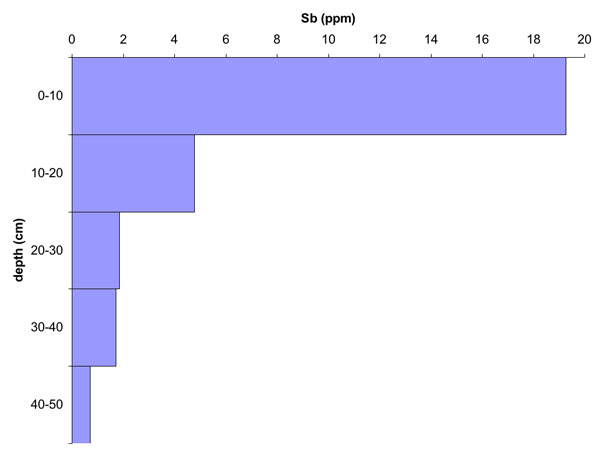
The Macleay floodplain (~600 km2) has been affected by flood-derived contaminated sediments deposited over the past century. Approximately 90% of the floodplain area contains Sb values above the local background value of 0.27 ppm, with As also exceeding the local background value. Approximately 8% of modern swamp environments and 6% of modern levee environments have Sb and As values exceeding current national soil ecological investigation levels of 20 ppm. Maximum Sb values (up to 39.4 ppm) occur in the swamps. Sediment cores taken from many locations in the most contaminated regions show maximum Sb concentrations in the top 20 cm, consistent with modern deposition. Mobilisation of Sb and As into water and plants can occur by redox and pH changes due to acid sulphate soil processes and management practices.
New Zealand examples
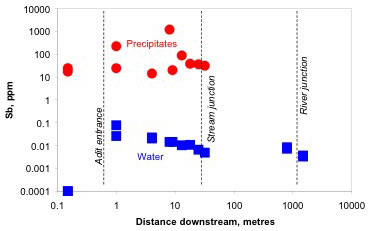
Vein-hosted gold mineralisation at Reefton is accompanied by arsenopyrite and minor stibnite and occurs in metasedimentary rocks. At Endeavour Inlet, the metasedimentary host rocks contain stibnite-rich veins. Pyrite content is low and mine drainages have circumneutral pH. Immediately downstream of mines, HFO containing high concentrations of Sb and As is precipitated, leading to downstream attentuation. The bulk distribution coefficient (Kd) for Sb and As absorption on to HFO ranges from ~103 to 105 and is similar to that determined at Hillgrove.
Antimony bio-availability
In the riparian environment of the Bakers Creek-Macleay River system, there is evidence of strong uptake of Sb (and to a lesser extent, As) into common plant species. Distributions in plants closely mimic those of corresponding stream sediments, although values are typically 1-2 orders of magnitude lower. Sb values in plants are commonly 3-100x background values for over 50 km downstream of the Hillgrove mines. Similarly, uptake of Sb occurs in aquatic alga with values intermediate between corresponding stream waters and sediments, and up to 2 orders of magnitude above background values.
Riparian vegetation (e.g. "Persicaria; "Plants 1" above) and alga (above) create pathways for fluvial cycling of Sb, including into invertebrate and vertebrate fauna. On the Macleay floodplain ("Plants 2" above), potential mobility of Sb and As can occur due to uptake from sediments into pasture species, acid sulphate soils processes and variable flooding regimes, leading to pH and redox changes. The extent of transfer of Sb and As into grazing animals (cattle), commercial crops and fish remains unknown in the Macleay catchment.
Experimental Data
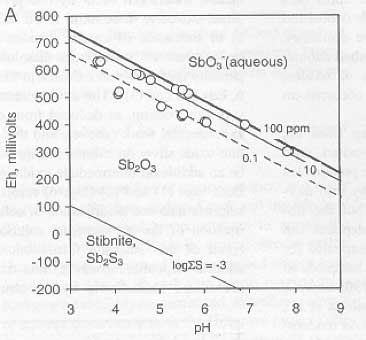
A. Eh-pH plot of theoretical solubility of the common Sb oxide, valentinite, with superimposed experimental data (red dots) from dissolution of Otago stibnite. The maximum solubilities obtained (37 mg/L Sb) correspond closely with field measurements of Sb solubility in Hillgrove mine waters (55 mg/L).
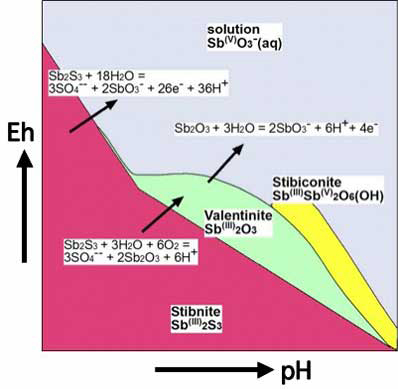
B. Spatial relationships among stibnite, Sb oxides and dissolved Sb in an oxidised surficial environment with chemical equations linking different Sb species.
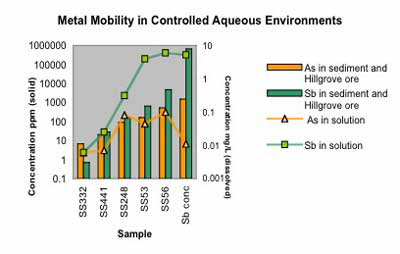
C. Graph of dissolved Sb and As concentrations in natural stream water, equilibrated for 32 days at 20°C with stream sediments containing from 7 ppm Sb to 4640 ppm Sb and with Hillgrove stibnite concentrate (66% Sb). Maximum dissolved Sb concentrations attain 5-6 mg/L and are 1-2 orders of magnitude greater than those of As.
Implications and Conclusions
Liberation of Sb (and As) into the environment is common from orogenic Sb-bearing ore deposits containing stibnite and Sb-bearing arsenopyrite.
Chemical mobilisation in solution and physical transport of mine wastes can lead to extensive dispersion of Sb and contamination of stream water and sediments, along with uptake into riparian vegetation and aquatic alga. Potentially, aquatic and terrestrial fauna can be affected with implications for bio-accumulation up the food chain.
The solubility of Sb is considerable in natural, circumneutral, oxidising fluids. It can also be enhanced by changes in pH and redox (e.g. (e.g. acid sulphate soil processes, mine waste treatments, land management techniques).
There is little that can be done practically and economically to catchments that have been subject to widespread historic contamination by Sb (and As). More needs to be discovered on biogeochemical pathways and toxicology, leading to development of appropriate management strategies.
References
Published papers from this work:
- Ashley et al., 2003: Journal of Geochemical Exploration, 77, 1-14.
- Craw et al., 2004: Applied Earth Science (TransIMM), 113, B3-B10.
- Wilson et al., 2004: Journal of Geochemical Exploration, 84, 127-139.
- Tighe et al., 2005: Science of the Total Environment, in press (available on-line).
Acknowledgements
Support for this project was given by NSW Department of Primary Industries, CSIRO Land and Water, and the Universities of New England and Otago. We would also like to thank the contributions of Ben Graham, Ben Wolfenden, Deb Chappell, Nat Wlson, Damian Walls, Lawrie Duck, Andrew Mundell, Peter Lockwood and Susan Wilson.
Stephen Read converted the poster into this web feature in May 2005. Some figures are linked to higher resolution versions - click on an image to see whether a clearer version is available.
Related
Introduction
Overview: Geological setting for Metals in the New Zealand environment
- Epithermal Gold
- Mesothermal Gold
- Acid Rock drainage
- Coal
- Metals in groundwater
- Alluvial Gold
- Northland Mercury
- Hot Springs (in heavy metals)
Metalloids
- Arsenic
- Antimony
- Antimony mobilisation from mineral deposits in schist and greywacke
- Mobilisation and dispersal of antimony from antimony-gold mineral deposits
- NZTEG 2008 - Antimony in the New Zealand environment