Mine tailings resulting from orogenic gold processing commonly form arsenic (As)-enriched environments. A result of this is that they draw environmental attention in modern gold mining settings and significant effort in long-term rehabilitation. The Alexander River mine processing site in the Reefton area of Westland provides an opportunity to examine the natural rehabilitation of As-enriched tailings that have been left undisturbed for >70 years in a humid environment. These tailings comprise a ~1 m thick package of finely laminated (mm-scale) sediments that were manually accumulated on a terrace downstream of the gold extraction plant. The tailings sediments are As-rich (up to 5000 mg/kg) due to relict arsenopyrite and As-bearing pyrite. Diagenetic Ca-Fe arsenate (tentatively identified as yukonite) and As-bearing iron oxyhydroxide have formed during localised oxidation. There was sufficient calcite in the tailings to maintain circumneutral pH in the tailings despite sulphide oxidation. The historical tailing impoundment is now covered by grass, ferns and shrubs, with beech and rimu forest covering several metres at the margins. Leaf analyses indicate that the vegetation is absorbing only minor As. Therefore, the tailings substrate has not halted natural vegetation re-colonisation of this As-rich historical site.
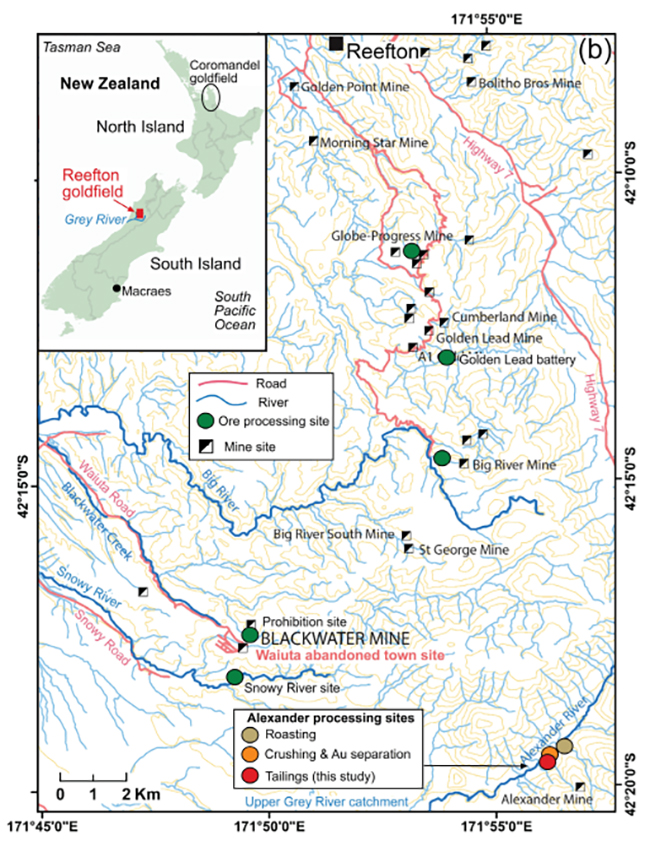
Figure 1. Location of the Alexander tailings deposit in the upper Grey River catchment. (a) Location of the hosting Reefton goldfield of western South Island; and (b) Location of tailings (red circle) immediately downstream of the Alexander mine processing system.
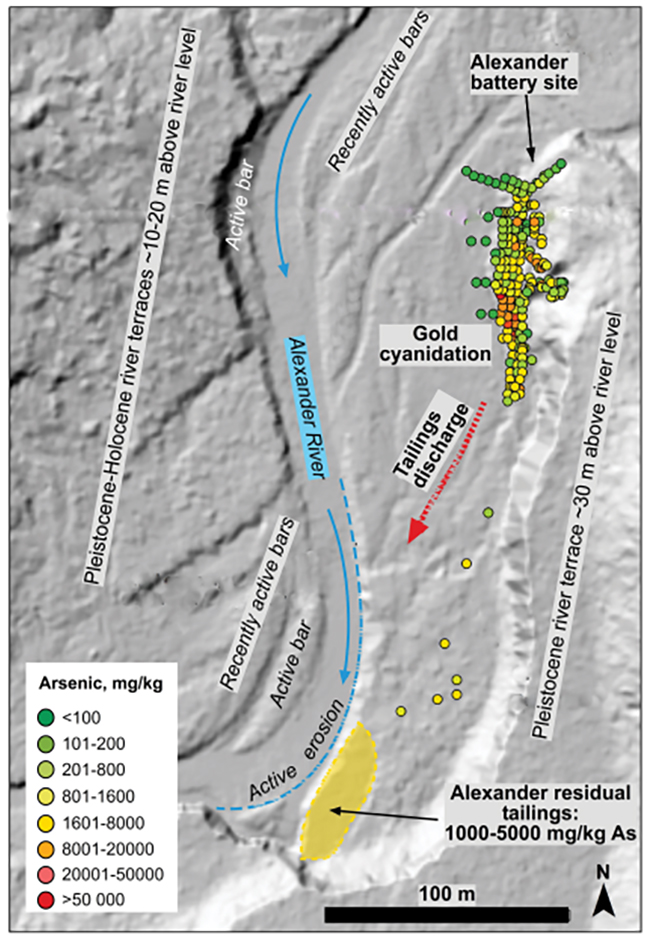
Figure 2. LIDAR image (from LINZ) of the dynamic fluvial environment of the Alexander crushing battery and cyanidation site, and downstream tailings. The point arsenic analyses clustered around the gold cyanidation plant were made by fp-XRF and taken from Malloch et al. (2017); the rest of the data were obtained in the present study.
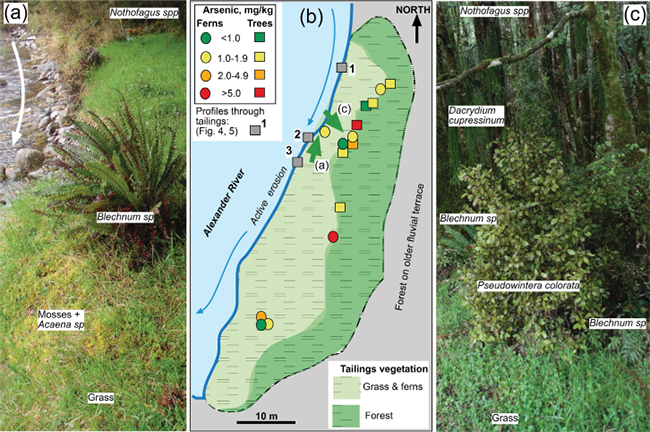
Figure 3. Present appearance of the Alexander tailings on a low terrace of the Alexander River. (a) Eroding river bank, grassed tailings surface, and forested tailings in background. (b) Sketch map of the whole tailings site, showing the distribution of grassed tailings and forested tailings, with directions of photos in a and c (green arrows). Ranges of As contents of plant leaves are indicated. (c) Forest edge on the tailings, with plant species analyses for As indicated.
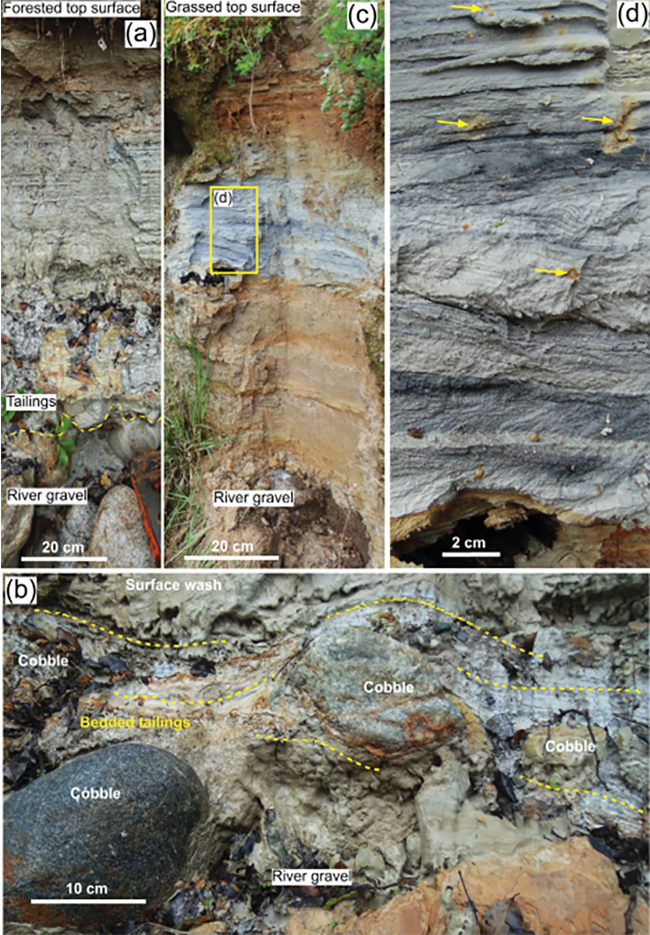
Figure 4. Field photographs of the base and central portions of the Alexander tailings exposed in the eroding river bank. (a) Base of the tailings deposit on river gravel. (b) Close view of the base of the tailings, showing bedding (yellow dashed lines) draped around cobbles of the gravel substrate. (c) Full section through tailings, showing differential oxidation (brown). (d) Close view of the middle portion of c, showing details of the bedding in tailings. Darker layers have finer particles than lighter layers, and are less oxidised. Small oxidation patches (yellow arrows) result from localised holes penetrating the tailings (possibly earthworms and/or rootlets).
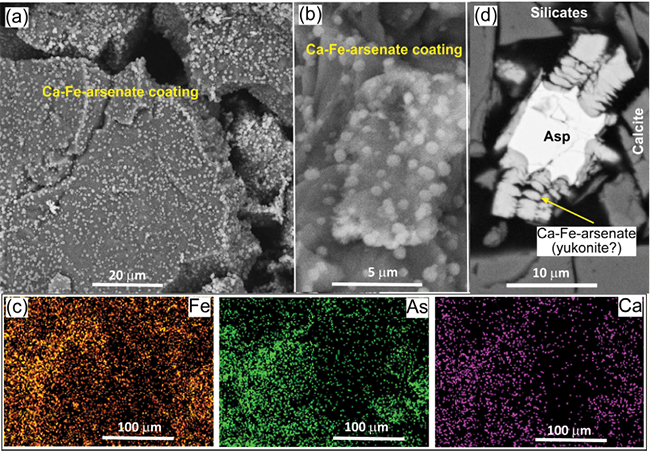
Figure 5. SEM images of Ca-Fe-arsenate coatings on silicate clasts in weakly oxidised tailings. (a) Backscatter electron image of silicate clasts (grey) with scattered amorphous Ca-Fe-arsenate precipitates. (b) Close view of the coating on a silicate clast. (c) Element maps showing Fe, As and Ca. Highest element contents are brightest. (d) SEM backscatter electron image of incipiently oxidised tailings showing an arsenopyrite clast (Asp) with an alteration halo of Ca-Fe-arsenate, probably yukonite.
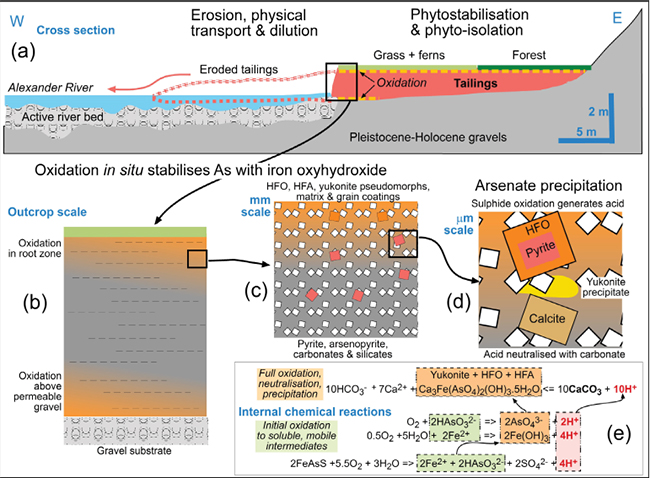
Figure 6. Summary of observations and inferences on the environmental stability of the Alexander tailings over the past 90 years. (a) Cross section shows inferred extent of erosion of the original tailings deposit by the eastward- migrating Alexander River channel. (b) Sketch of progressive incursion of oxidation fronts from above and below. (c) Sketch at particle scale of the downward-migrating oxidation front. (d) Sketch at the mineral particle scale of the downward-migrating oxidation front. (e) Summary chemical equations for oxidation of arsenopyrite, mobilisation of soluble As and Fe, followed by neutralisation of acid by calcite and precipitation of yukonite.