Arsenic in fresh schist rocks and sediments
All rocks contain some arsenic (As), and typical freshly-exposed (unweathered) Otago Schist rocks contain slightly more than most. Fresh Otago Schist exposed in mountains to the west of Cromwell and in deep gorges elsewhere in Otago, has arsenic concentrations between 5 and 20 mg/kg, with some rocks having up to 30 mg/kg As. These are still low levels, but the upper levels of this range overlap with maximum recommended levels for soils (20 mg/kg). Freshly-eroded sediments, including silts, sands and gravels that are common to the west of Cromwell, also have the same range of As concentrations as the rocks from which they were derived.
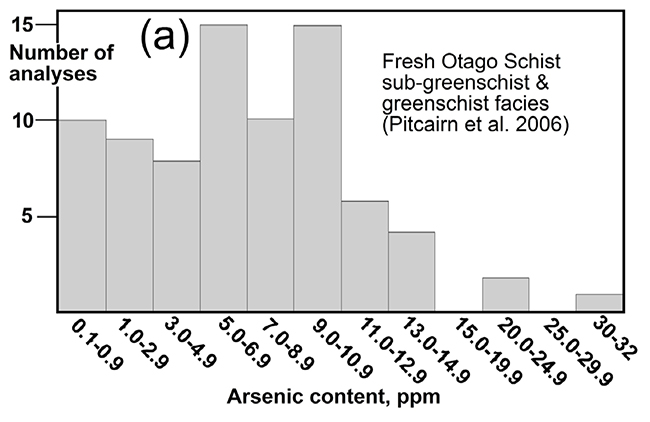
Histogram of arsenic concentrations (ppm = mg/kg) of typical Otago Schist rocks. From Blake et al. (2019), with data from Pitcairn et al. (2006).
The arsenic in fresh schist occurs mainly in small mineral grains (about 10 microns; one hundredth of a millimetre) scattered through the rocks. The most common mineral containing As is the iron sulphide mineral pyrite (FeS2; “fools gold”), and there is also some As in the related iron sulphide mineral pyrrhotite (FeS). This As is typically dispersed through the iron sulphide mineral grains at concentrations from 100 to 5000 mg/kg. In addition, separate arsenic sulphide minerals can also occur with the pyrite or pyrrhotite. Arsenopyrite (FeAsS) occurs with pyrite in hydrothermal gold deposits, but is not common in ordinary schist. Instead, the most common arsenic sulphide mineral is cobaltite (CoAsS). As the formula suggests, this mineral has a very high proportion of arsenic (about 45%), but the grains are very small and widely disseminated through the rocks.

Patches of very fine grained pyrite (framboidal = raspberry-shaped) from rocks at the northeastern edge of the Otago Schist (Manuherikia gorge, Fiddlers Flat). The pyrite contains disseminated As.
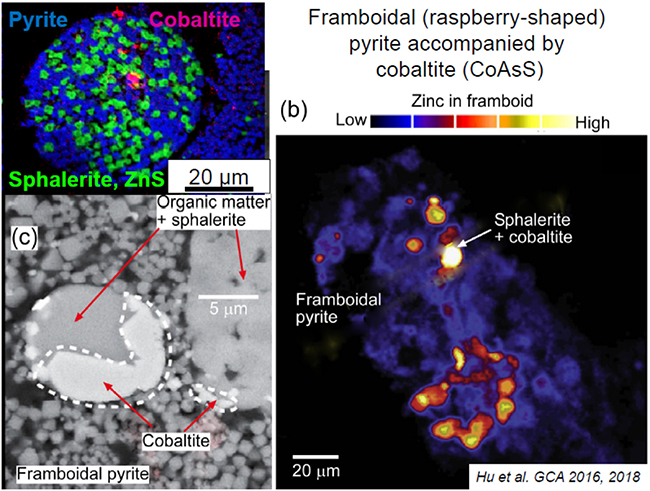
Images of framboidal pyrite with rare small grains of cobaltite (CoAsS). Modified from Hu et al. 2016, 2018).

Microscopic image of a grain of pyrrhotite (FeS) surrounded by the dominant Otago Schist minerals quartz (Qtz), muscovite mica (Ms), and epidote (Ep). The pyrrhotite contains minor nickel and cobalt, as analysed at the two yellow spots, but no arsenic. Instead, arsenic occurs in cobaltite (CoAsS; red spot) with small amounts of nickel and antimony. Modified from Pitcairn et al. (2010).
Arsenic in weathered (oxidised) schist rocks and sediments
Schist rocks exposed at the surface for any length of time become weathered and turn from grey to brown as the iron content is oxidised. The sulphide minerals that contain As are unstable in this oxidation process, and they break down to a range of weathering products. Oxidation at or near the surface results in formation of oxidised As minerals such as scorodite (FeAsO4.2H2O), which can form green coatings on some dry rock surfaces.
More commonly, some of the brown colour of the weathered schist is a result of oxidation of pyrite or pyrrhotite, which breaks down to “rusty” stains on rock surfaces. This rusty material, called hydrated iron oxide or Fe oxyhydroxide can also form very fine grained particles in groundwater, causing stains on surfaces wherever it passes. The hydrated iron oxide has a strong chemical ability to attract and bind arsenic (= adsorption) in these oxidised environments. Hence, hydrated iron oxide stains and particles in groundwater typically have higher As concentrations than typical rocks. These As concentrations of Fe oxyhydroxide can range from about 100 mg/kg up to tens of thousands of mg/kg. Many engineered water treatment systems that extract As use this property of Fe oxyhydroxide to attract As from the water on to solid surfaces.
Iron oxide adsorption (Wikipedia)
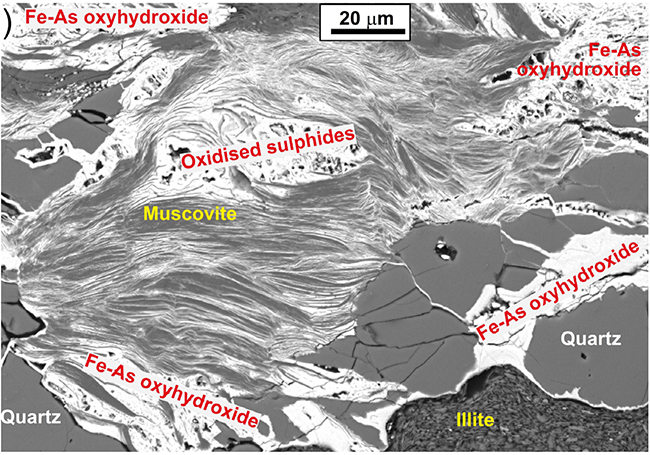
Scanning electron microscopic view of oxidised schist with muscovite mica and quartz that have been extensively stained by As-bearing iron oxyhydroxide (white). From Blake et al. (2019).
Because arsenic has been released from the rocks into groundwater during these oxidation processes, the rocks or sediments consequently have lowered As concentrations (typically 5–10 mg/kg). Soils have undergone more extensive weathering during formation, and As concentrations are commonly about 5 mg/kg or even lower. However, where groundwater has caused staining by iron oxyhydroxide, As concentrations can be locally higher than the original rocks.
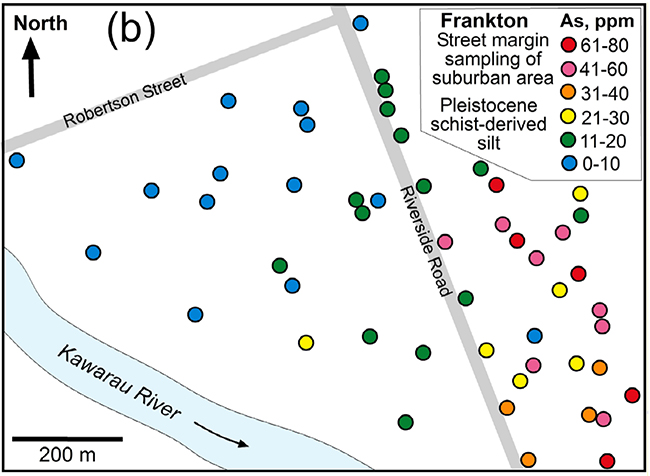
Arsenic concentrations in fresh schist sediments (glacial origin) that are undergoing oxidation at Frankton (Queenstown). Elevation of As concentrations probably resulted from dissolving of disseminated As-bearing sulphide minerals, with reprecipitation of some of that As on iron oxyhydroxide stains. From: Blake et al. (2019).
Intermediate levels of oxidation
At the base of the oxidation zone (typically 5–50 metres below surface), where rocks are only partially oxidised, there is little or no formation of iron oxyhydroxide and consequently no adsorption of As on to solid surfaces. Instead, the arsenic that is released from the rocks is highly soluble in groundwater. This is the principal zone of concern for elevated dissolved As in drinking water. There is a mineral, arsenolite (As2O3) which can form in this zone, but dissolved As must reach extremely high concentrations (hundreds of mg/L) before that mineral will form.
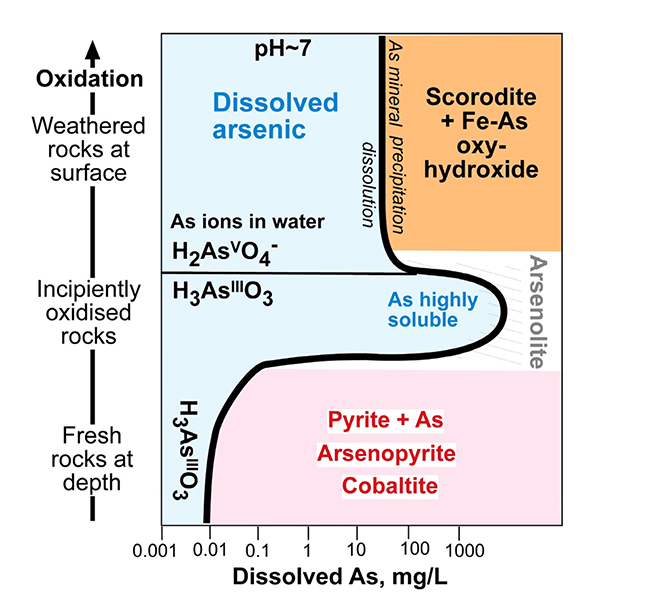
Arsenic solubility geochemical diagram, showing changes in solubility of As in groundwater through the weathering profile below the surface (heavy black line bounding blue solubility field). Principal stable minerals at different levels are shown on right side of the solubility line. Modified from Blake et al. 2019).
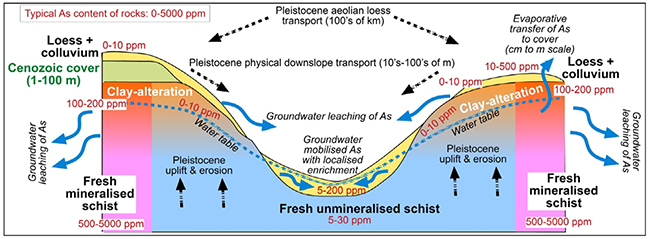
Cartoon showing the geological and hydrogeological features relevant to arsenic in the Otago Schist weathering zone, for typical schist (centre) and for mineralised schist at gold mines (edges of diagram). Concentrations (ppm, parts per million) are for rocks ( = mg/kg) and waters (= mg/L). From Blake et al. 2019).
References and further information on Otago Schist arsenic
Blake F, Grant K, MacKenzie D, Scott, J, Craw D. 2019 Surficial arsenic redistribution above gold-mineralised zones in East Otago, New Zealand. New Zealand Journal of Geology and Geophysics 62: 573-587.
Hu, S.-Y., Evans, K., Fisher, L., Rempel, K., Craw, D., Evans, N. J., Cumberland, S., Robert, A., Grice, K. 2016. Associations between sulfides, carbonaceous material, gold and other trace elements in polyframboids: Implications for the source of orogenic gold deposits, Otago Schist, New Zealand. Geochimica et Cosmochimica Acta 180: 197-213.
Hu, S-Y, Evans K, Rempel K, Guagliardo P, Kilburn M, Craw D, Grice K, Dick J. 2018. Sequestration of Zn into mixed pyrite-zinc sulfide framboids: a key to Zn cycling in the ocean? Geochimica et Cosmochimica Acta 241:95-107.
Large R, Thomas H, Craw D, Henne A, Henderson S 2012. Diagenetic pyrite as a source for metals in orogenic gold deposits, Otago Schist, New Zealand. New Zealand Journal of Geology and Geophysics 55: 137-149.
Pitcairn, I.K., Teagle, D.A.H., Craw, D., Olivo, G.R., Kerrich, R., Brewer, T.S. 2006. Sources of metals and fluids in orogenic gold deposits: insights from the Otago and Alpine Schists, New Zealand. Economic Geology 101: 1525-1546.
Pitcairn, I K, Olivo, G R, Teagle, D A H & Craw, D. 2010. Sulfide evolution during prograde metamorphism of the Otago and Alpine Schists, New Zealand. The Canadian Mineralogist 48: 1267-1295.