Background information on the Munida Time-Series Transect
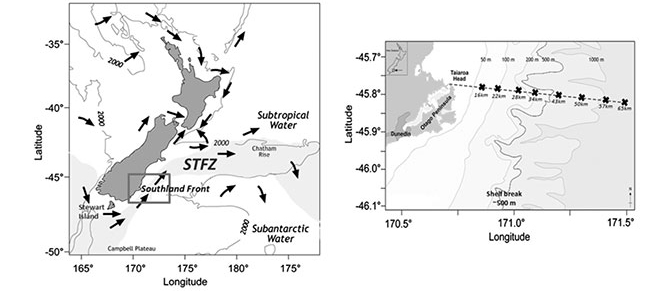
Fig 1. The Subtropical Frontal Zone (STFZ) east of New Zealand (left). Image reproduced from Jones et al (2013). The STFZ (indicated in grey) represents the transitional zone. The near-coastal portion of the STFZ off the coast of Otago (indicated in box) is designated as the Southland Front. The inset (right) depicts the study area located off the Otago Peninsula and the 65 km Munida Time-Series Transect. The edge of the continental shelf tracks along the 500 m isobath, as highlighted in inset.
The Munida Time-Series Transect (Fig 1) was established in 1998 by Kim Currie and Keith Hunter to study the role of oceanic waters in the South Pacific in the uptake of atmospheric CO2. It also aimed to understand the seasonal, interannual and long-term variability of carbonate chemistry within these waters. The transect originates from Otago, in the south east coast of New Zealand, and covers 65 km crossing neritic, subtropical and subantarctic surface waters. Sampling takes place approximately every 2 months and includes sampling of surface waters (2 m) at eight stations in addition to a 'deep' mesopelagic (500 m) sample in the farthest station. The Munida Time Series is a recognised oceanographic CO2 reference site representing the longest time series of iron speciation along a coastal shelf area, and boasting the Southern Hemisphere's longest-running ocean record of pH measurements.
The Munida transect consists of 8 stations to a distance of around 65 km from the Otago Coast (Fig 1). Starting in near shore station, the transect crosses Neritic waters (coastal water with riverine influence), Subtropical waters (STW), and Subantarctic waters (SAW). In doing so, it crosses the Subtropical Frontal Zone, which separates the STW from the SAW. The main aim is to compare the spatial variability along the surface waters of the front to the vertical (in-depth) zonation characteristic of oceanic microbial communities.
As a result of prior work in the Munida Time Series we know that SAW are a sink for atmospheric carbon dioxide (Currie et al., 2011), with seasonal cycles of DIC (dissolved inorganic carbon) primarily driven by net community production (Brix et al., 2013; Jones et al., 2013) with modification by the annual cycle of sea surface temperature. Dissolved Fe concentrations decrease seaward and Fe is fully complexed by strong organic ligands (Sander et al. 2015). The much lower Fe concentration in SAW is characteristic of the high-nutrient low chlorophyll (HNLC) waters that are associated with Fe-limited phytoplankton population and productivity (Boyd et al., 1999; Bradford-Grieve et al., 1997; Tian et al., 2006).
Why is the Munida transect unique?
The Munida transect has several features that make it unique aside from access to historic information on measurements along the transect.
Oceanic fronts
Oceanic fronts are areas where distinct water masses meet creating enhanced horizontal gradients of physicochemical properties (e.g. temperature, salinity) (Belkin, 2003). These mixing zones are boundaries between different oceanic masses. At Munida, the macronutrient-limited (e.g. N, P) Subtropical waters (STW) encounter the micronutrient-limited (e.g. Fe) Subantarctic waters (SAW) thus forming a front (Baltar 2016). This allows us to study two globally relevant water masses, as well as the frontal zone separating them, within a single transect.
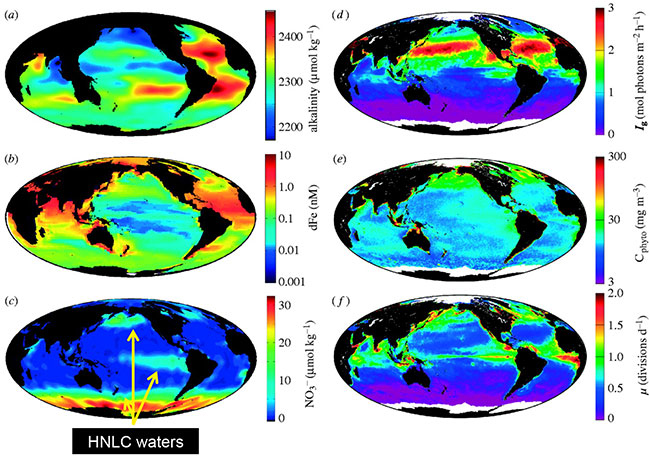
Fig 2. Global distributions of (a) annual average total alkalinity as an index of inorganic carbon concentration (data from Key et al., 2004), (b) boreal summer mixed layer dissolved iron (dFe) concentration (data from Moore & Braucher, 2007), (c) July surface nitrate (Levitus climatology), (d) July median mixed layer growth irradiance (Ig), (e) July surface phytoplankton carbon (Cphyto) biomass, and (f) July surface phytoplankton growth rates (m). Data for (d–f) are from http://www.science.oregonstate.edu/ocean.productivity/. Image reproduced from Behrenfeld (2008).
High-nutrient, low chlorophyll (HNLC) waters
HNLC regions are areas of the ocean where micronutrients (especially iron) limit biological productivity despite readily available macro nutrients (Fig 2), leading to limited phytoplankton blooms. These HNLC regions cover 30% of the world's oceans and share key features and community responses (Martin et al., 1994; Coale et al., 1996; Boyd et al., 2000; Tsuda et al., 2003; Boyd et al., 2004). The Subantarctic waters within Munida (representative of the Southern Ocean) are of particular importance because of their role in C transport into the deep ocean (Broecker and Henderson, 1998; Sarmiento et al., 1998).
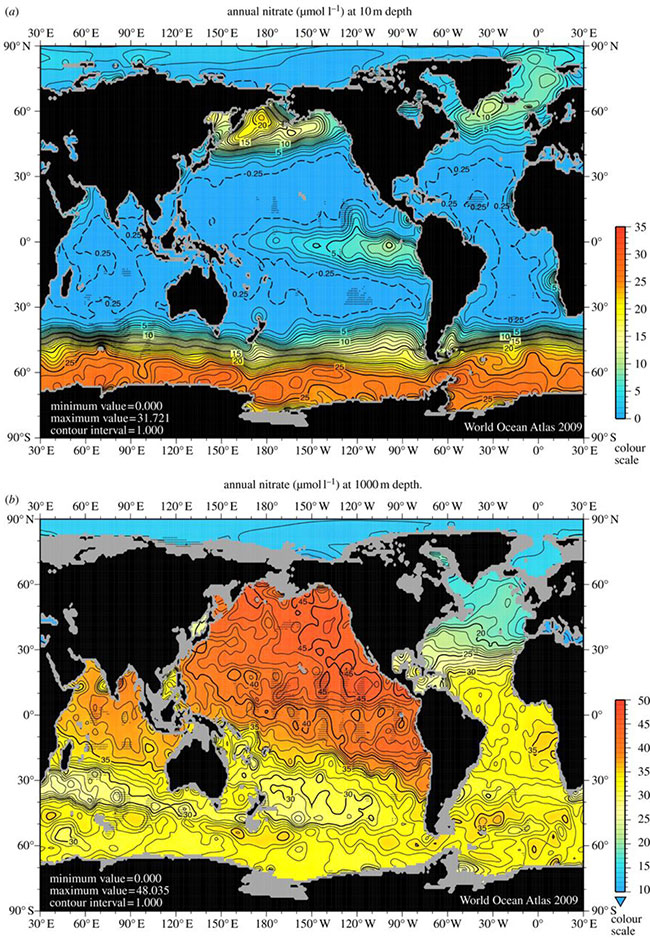
Fig 3. Nitrate concentrations in (a) surface waters of the ocean and (b) at 1000 m depth. Image reproduced from Voss (2013).
Deep (mesopelagic) water
Most oceanographic research has focused on the shallow sunlit layers of the ocean with much less known about the deep dark ocean, despite deep water representing the largest habitat in the biosphere. These deep waters harbour an estimated ~75% of the prokaryotic biomass and 50% of the prokaryotic production of the global ocean (Arístegui et al., 2009). This lack of research in the dark ocean is probably based on the presumption that it is an homogenous system with low microbial activity. However, recent studies are showing that it is a more dynamic system in terms of spatial and seasonal variability in microbial abundance and activity (Sherry et al., 2002; Tanaka and Rassoulzadegan, 2002; 2004; Gasol et al., 2009; Tamburini et al., 2009; Baltar et al., 2010a,b; Baltar et al., 2012). MOTS is among the few time-series collecting microbial data from the dark ocean (mid-mesopalagic layers), and the only one to do so in HNLC SAW.
Access
While any of the above features would make a site interesting, Munida combines all. Further, because of its location it has the unique distinction of being accessible from shore and can be sampled within a single day trip using a research vessel moored at the Portobello Lab (Fig 4).

Fig 4. Polaris II research vessel used for sampling.
References cited
- Sherry, N.D., Imanian, B., Sugimoto, K., Boyd, P.W., and Harrison, P.J. (2002). Seasonal and interannual trends in heterotrophic bacterial processes between 1995 and 1999 in the subarctic NE Pacific. Deep-Sea Res. II 49, 5775-5791.
- Brix, H., Currie, K.I., Mikaloff Fletcher, S., 2013. Seasonal variability of the carbon cycle in subantarctic surface water in the South West Pacific. Global Biogeochemical Cycles 27, 1-12.
- Boyd, P., LaRoche, J., Gall, M., Frew, R., Mckay, R.M., 1999. Role of iron, light, and silicate in controlling algal biomass in subantarctic waters SE of New Zealand. J. Geophys. Res. 104, 13395–13408.
- Bradford-Grieve, J.M., Chang, F.H., Gall, M., Pickmere, S., Richards, F., 1997. Size-fractionated phytoplankton standing stocks and primary production during austral winter and spring 1993 in the Subtropical Convergence region near New Zealand. N. Z. J. Mar. Freshw. Res. 31, 201–224.
- Tian, F., Frew, R.D., Sander, S., Hunter, K.A., Ellwood, M.J., 2006. Organic iron(III) speciation in surface transects across a frontal zone: the Chatham Rise. N. Z. J. Mar. Freshw. Res. 57, 533–544.
- Jones, K., Currie, K.I., McGraw, C.M., Hunter, K.A., 2013. The effect of coastal processes on phytoplankton biomass and primary production within the near-shore Subtropical Frontal Zone. Estuarine, Coastal and Shelf Science 124, 44-55.
- Belkin I (2003). Front. Interdisciplinary Encyclopedia of Marine Sciences, edited by Nybakken JW, Broenkow WW, Vallier TL: 433-436.
- Morales SE, Meyer M, Currie K, Baltar F. (2018). Are oceanic fronts ecotones? Seasonal changes along the subtropical front show fronts as bacterioplankton transition zones but not diversity hotspots. Environ Microbiol Rep. e-pub ahead of print, doi: 10.1111/1758-2229.12618.
- Behrenfeld MJ, Halsey KH, Milligan AJ. (2008). Evolved physiological responses of phytoplankton to their integrated growth environment. Philosophical transactions of the Royal Society of London Series B, Biological sciences 363: 2687–2703.
- Baltar F, Currie K, Stuck E, Roosa S, Morales SE. (2016). Oceanic fronts: transition zones for bacterioplankton community composition. Environ Microbiol Rep 8: 132–138.
- Martin JH, Coale KH, Johnson KS, Fitzwater SE, Gordon RM, TANNER SJ, et al. (1994). Testing the Iron Hypothesis in Ecosystems of the Equatorial Pacific-Ocean. Nature 371: 123–129.
- Coale KH, Johnson KS, Fitzwater SE, Gordon RM, Tanner S, Chavez FP, et al. (1996). A massive phytoplankton bloom induced by an ecosystem-scale iron fertilization experiment in the equatorial Pacific Ocean. Nature 383: 495–501.
- Boyd PW, Watson AJ, Law CS, Abraham ER, Trull T, Murdoch R, et al. (2000). A mesoscale phytoplankton bloom in the polar Southern Ocean stimulated by iron fertilization. Nature 407: 695–702.
- Tsuda A, Takeda S, Saito H, Nishioka J, Nojiri Y, Kudo I, et al. (2003). A mesoscale iron enrichment in the western subarctic Pacific induces a large centric diatom bloom. Science 300: 958–961.
- Boyd PW, Law CS, Wong CS, Nojiri Y, Tsuda A, Levasseur M, et al. (2004). The decline and fate of an iron-induced subarctic phytoplankton bloom. Nature 428: 549–553.
- Baltar F, Currie K, Meyer M, Verdugo P. (2016). Proportion of marine organic carbon present in self-assembled gels along the subtropical front and its increase in response to reduced pH - ScienceDirect. Marine Chemistry.
- Baltar F, Stuck E, Morales S, Currie K. (2015). Bacterioplankton carbon cycling along the Subtropical Frontal Zone off New Zealand. Progress in Oceanography 135: 168–175.
- Currie, K.I., Reid, M.R., Hunter, K.A., 2011. Interannual variability of carbon dioxide drawdown by subantarctic surface water near New Zealand. Biogeochemistry 104, 23-34.
- Sylvia G. Sander, Feng Tian, Enitan B. Ibisanmi, Kim I. Currie, Keith A. Hunter, Russell D. Frew .Spatial and seasonal variations of iron speciation in surface waters of the Subantarctic front and the Otago Continental Shelf. Marine Chemistry 173 (2015) 114–124
- Katherine N. Jones, Kim I. Currie, Christina M. McGraw, Keith A. Hunter. The effect of coastal processes on phytoplankton biomass and primary production within the near-shore Subtropical Frontal Zone. Estuarine, Coastal and Shelf Science 124 (2013) 44e55
- Tanaka, T., and Rassoulzadegan, F. (2002). Full-depth profile (0-2000 m) of bacteria, heterotrophic nanoflagellates and ciliates in the NW Mediterranean Sea: vertical partitioning of microbial trophic structures. Deep Sea Res. II 49, 2093-2107.
- Tanaka, T., and Rassoulzadegan, F. (2004). Vertical and seasonal variations of bacterial abundance and production in the mesopelagic layer of the NW Mediterranean Sea: bottom-up and top-down controls. Deep Sea Res. I 51, 531-544.
- Gasol, J.M., Alonso-Sáez, L., Vaqué, D., Baltar, F., Calleja, M.L., Duarte, C.M., and Arístegui, J. (2009). Mesopelagic prokaryotic bulk and single-cell heterotrophic and community composition in the NW Africa-Canary Islands coastal-transition zone. Progress In Oceanography 83, 189-196.
- Baltar, F., Arístegui, J., Gasol, J.M., and Herndl, G.J. (2010a). Prokaryotic carbon utilization in the dark ocean: growth efficiency, leucine-to-carbon conversion factors, and their relation. Aquatic Microbial Ecology 60, 227-232.
- Baltar, F., Arístegui, J., Gasol, J.M., Lekunberri, I., and Herndl, G.J. (2010b). Mesoscale eddies: Hotspots of prokaryotic activity and differential community structure in the ocean. The ISME Journal 4, 975-988.
- Baltar, F., Arísstegui, J., Gasol, J.M., and Herndl, G.J. (2012). Microbial Functioning and Community Structure Variability in the Mesopelagic and Epipelagic Waters of the Subtropical Northeast Atlantic Ocean. Applied and Environmental Microbiology 78, 3309-3316.
- Arístegui, J., Gasol, J.M., Duarte, C.M., and Herndl, G.J. (2009). Microbial Oceanography of the dark ocean's pelagic realm. Limnology and Oceanography 54, 1501-1529.
- Voss M, Bange HW, Dippner JW, Middelburg JJ, Montoya JP, Ward B. (2013). The marine nitrogen cycle: recent discoveries, uncertainties and the potential relevance of climate change. Philosophical Transactions of the Royal Society B: Biological Sciences 368: 20130121.