
This year's winner of the Translational Research Grant will use the $50,000 prize money to develop a device to improve treatment for pre-stage cervical cancer.
Winners of the Translational Research Grant 2023 will use the $50,000 prize money to develop a device to improve treatment for pre-stage cervical cancer.

Dr Noelyn Hung.
Currently, women can opt for either surgical intervention, or pharmaceutical intervention. While pharmaceutical intervention is less invasive, it can require multiple clinical appointments each week.
The winning team is made up of University of Otago’s Associate Professor Jaydee Cabral, a biomedical scientist and bioengineer of the Department of Microbiology & Immunology and the Centre of Bioengineering & Nanomedicine; Dr Noelyn Hung, a clinical pathologist of the Department of Pathology; and Dr Adel Mekhail, a gynaecologist and obstetrician of Te Whatu Ora Health NZ – Southern at the Dunedin hospital.
The grant of $50,000 will advance the teams’ planned in vitro work and inform any potential intellectual property protection pathway.
Otago Innovation chief executive David Christensen says if successful, Otago Innovation will invest into further commercialisation to translate the team’s approach into clinical practice.
“There is certainly a great need for this exciting research.”
The team plans on improving a pharmaceutical intervention and will use the money to advance the testing of one of their concepts.
Figures from the Ministry of Health show there was an average of 171 women diagnosed with cervical cancer each year, which is 6.2 per every 100,000 females, between 2015 and 2020, while there were 53 deaths, which is 1.6 per every 100,000 females, between 2015 and 2018.
However, Associate Professor Cabral says the incidence of high-grade changes is much higher.

Associate Professor Jaydee Cabral.
“What we are trying to achieve is to reduce the need for surgery as the sole treatment for these changes, especially in young women during their reproductive years when the integrity of the genital tract can be compromised by surgical treatment.”
Associate Professor Cabral and the rest of the team are trying to use an immunomodulator drug which has been proven effective in treating high-grade changes.
“We hope to deliver this drug as a one-time treatment option versus needing multiple repeat applications by the doctor.”
Associate Professor Cabral says it might take the team 1 to 2 years to demonstrate proof-of-concept in vitro.
“We would then seek additional funding for large animal in vivo trials which would take another year. We would see IP protection and further investment to move to clinical testing.”
Once the treatment was validated clinically, the team would seek regulatory approval for its device. In total, it may take around 10 years to produce a commercially available product.
Dr Mekhail says while incidence and mortality rates from cervical cancer have decreased overall in Aotearoa New Zealand, Māori and Pacific peoples continue to have a higher incidence rate.
“Māori are also more than twice as likely to die from cervical cancer than Europeans. Cervical cancer is the second leading cause of death for Māori females aged 25 to 44 years.”
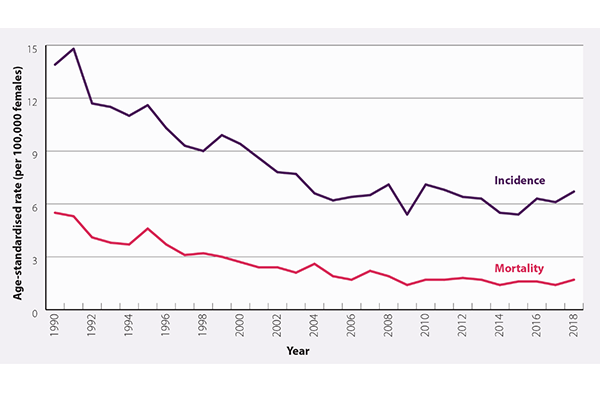
The incidence and mortality rates of cervical cancer in Aotearoa New Zealand per 100,000 women.
The starting age for the screening programme in Aotearoa New Zealand is 25. The smear test will be performed once every 3 to 5 years if the female tests negative for HPV, and once every year is she test positive. Dr Mekhail says HPV DNA is present in 99% of all cervical cancers, but infection alone in people with a competent immune system is not sufficient to cause invasive cervical cancer.

Dr Adel Mekhail.
“It is estimated that two-thirds of people – including males – become infected with HPV within three years of being sexually active, and up to 80% of people will be exposed to HPV at some stage in their lifetime.
“However, if the disease has progressed to high grade changes, the woman is referred to the colposcopy clinic.”
Three-yearly cervical screening attendance in New Zealand has been steadily declining over time, with an accelerated rate of decline taking place during the COVID-19 pandemic. Numbers went from 76% of eligible people in 2008 attending screening, to 67% attending in June 2022.
“There are also considerable inequities in cervical screening attendance; in June, 2022, the national three-year coverage was 57.7% for Asian, 55.7% for Pacific peoples and 54.9% for Māori, compared to 74.4% for European/Other.”
People living in low socioeconomic areas also have lower rates of attendance for cervical screening, Dr Mekhail says.
“Low rates of cervical screening attendance is concerning as most cervical cancers in New Zealand occur in people who are not regularly screened.”
Associate Professor Cabral says reducing the need for visits or surgical treatment by developing the team’s Modality of treatment could be a great way to help Māori and Pasifika women feel more comfortable attending smears and improving detection times.
Doctors could also provide culturally appropriate educational materials outlining the importance of cervical cancer screening, help to minimise embarrassment or vulnerability by discussing actions that could help Māori or Pasifika patients feel more comfortable such as bringing a support person, choosing their sample taker – gender, ethnicity, providing appropriate covering for the patient and pulling curtains around bed to ensure privacy.
Explaining each step of the process as it is carried out can also help make patients feel more relaxed, she says.
-Kōrero by internal communications adviser, Koren Allpress